Theoretical elucidation of the multi-functional synthetic methodology for switchable Ni(0)-catalyzed C–H allylations, alkenylations and dienylations with allenes†
Abstract
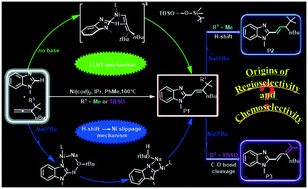
The Ni(0)-catalyzed coupling of benzimidazole with 1,1-disubstituted allenes represents a new strategy for achieving controllable C–H allylations, alkenylations and dienylations. To understand the detailed mechanisms and origins of the switchable selectivities, density functional theory (DFT) calculations were conducted. The results using a tBu-substituted allene demonstrate that the formation of the allylated product involves a Ni-catalyzed C–H activation mechanism through ligand-to-ligand-hydrogen transfer (LLHT) under base-free conditions. In contrast, a Ni/NaOtBu co-promoted C–H activation mechanism is newly proposed in the presence of NaOtBu, which is remarkably different from the previously reported literature. The novel mechanism emphasizes that NaOtBu abstracts the Ni-activated heterocyclic (ipso-C)H atom followed by turnover limiting Ni slippage, and subsequently the allylated product is generated after alkene insertion and protonation. The strong electrostatic attraction between Ni and heterocyclic ipso-C in the Ni slippage pre-intermediate is critical for facilitating the Ni slippage. Once formed, the allylated product, assisted by NaOtBu, further evolves into a more stable alkenylated isomer. Employing a (tert-butyldimethylsilyl)-ether substituted allene as the substrate, the NaOtBu-induced chemoselectivity for dienylation vs. alkenylation was also probed and it was found that the O(tBu)–H⋯O(Si) hydrogen bonding interaction in the C–O(Si) cleavage pre-intermediate remarkably weakens the adjacent C–O(Si) σ-bond, thereby resulting in an exclusive C–O(Si) cleaved dienylation product. Further theoretical predictions suggest that the chemoselectivity might be reversed by replacing tBu in NaOtBu by the withdrawing C(CF3)3 group.


 Please wait while we load your content...
Please wait while we load your content...