Understanding the effect of interfacial interaction on metal/metal oxide electrocatalysts for hydrogen evolution and hydrogen oxidation reactions on the basis of first-principles calculations†
Abstract
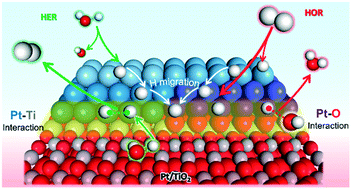
Understanding the interfacial interaction between metals (M) and transition metal oxide (TMO) supports is essential for the material design of high performance electrocatalysts. In this study, we constructed Pt/TiO2 composite models with interfacial Pt–Ti and Pt–O bonds respectively, to clarify the interface effect on electronic structures, species adsorption/desorption and migration properties, and HER and HOR mechanisms through first-principles calculations. Interfacial Pt–Ti metallic interaction causes more delocalized electrons on the Pt cluster, which strengthens H and OH adsorption, facilitating *H migration and H2O dissociation. Meanwhile interfacial Pt–O covalent interaction affects the localized electronic structure of Pt with deficient electrons, which results in optimum H adsorption/desorption and complicated *H migration processes. The interface sites and non-interface sites jointly provide an efficient H migration channel and reaction pathway. For the HER, the Pt–O interaction benefits the Tafel step, and the Pt–Ti interaction is favorable for the Volmer step. For the HOR, the Pt–O interaction contributes to a higher HOR activity and anti-oxidation ability than Pt–Ti. These results can serve as theoretical guidance for constructing high performance M/TMO electrocatalysts for the HER/HOR by regulating the interfacial bond type and proportion.


 Please wait while we load your content...
Please wait while we load your content...