Catalytic reductive deoxygenation of esters to ethers driven by hydrosilane activation through non-covalent interactions with a fluorinated borate salt†
Abstract
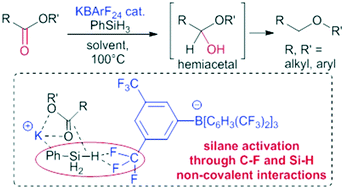
We report the catalytic and transition metal-free reductive deoxygenation of esters to ethers through the use of a hydrosilane and a fluorinated borate BArF salt as a catalyst. Experimental and theoretical studies support the role of noncovalent interactions between the fluorinated catalyst, the hydrosilane and the ester substrate in the reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...