Manganese(i) κ2-NN complex-catalyzed formic acid dehydrogenation†
Abstract
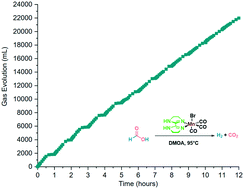
Hydrogen use as a sustainable energy vector is of growing interest and is the subject of current intensive research. We report herein an upgraded follow-up system for formic acid (FA) dehydrogenation (DH) catalyzed by [Mn(BisIm)(CO)3Br] (BisIm = 4,4′,5,5′-tetrahydro-1H,1′H-2,2′-bisimidazole). The latter belongs to a family of novel catalysts we developed, which are interesting since they bear easily accessible ligands from cheap, commercially available starting materials. The novel catalyst system allows for improved hydrogen generation and better catalyst turnover numbers (7883 after 12 cycles of FA dosage). In addition, a significantly lower CO content in the gas phase was also achieved (47 ppm). Extended investigation of the influence of the ligand structure on the catalytic performances confirmed that the non-bonding electron pair on the N–H moiety, on the 3rd position of each imidazoline ring, is crucial for the transformation.


 Please wait while we load your content...
Please wait while we load your content...