Nickel catalysed construction of benzazoles via hydrogen atom transfer reactions†
Abstract
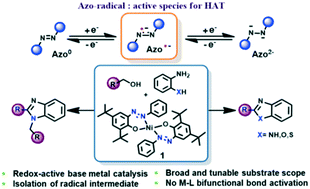
Herein we report a homogeneous, phosphine free, inexpensive nickel catalyst that forms a wide variety of benzazoles from alcohol and diamines by a reaction sequence of alcohol oxidation, imine formation, ring cyclization and dehydrogenative aromatization. A reversible azo/hydrazo couple, that is part of the ligand architecture steers both the alcohol oxidation and dehydrogenation of the annulated amine under fairly mild reaction conditions. Interestingly, both the alcohol oxidation and amine dehydrogenation steps are directly mediated by hydrogen atom transfer (HAT), which is greatly facilitated by the reduced ligand backbone. The kH/kD for the amine dehydrogenation step, measured at 60 °C is 5.9, fully consistent with HAT as the rate determining factor during this step. This is a unique scenario where two consecutive oxidation steps towards benzazole formation undergo HAT, which has been substantiated via kinetic studies, KIE determination and intermediate isolation.


 Please wait while we load your content...
Please wait while we load your content...