Alcohol amination over titania-supported ruthenium nanoparticles†
Abstract
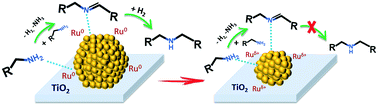
Metal nanoparticles for heterogeneous catalytic reactions are often supported on porous materials. The catalytic performance of supported catalysts usually depends on the size of the metal nanoparticles and catalytic supports. Selective synthesis of valuable primary amines is an important target in the modern industry. In this work, the catalytic performance of TiO2 supported Ru nanoparticles with sizes from 1.4 to 9.9 nm was investigated in both direct gas-phase amination of 1-butanol and liquid-phase amination of 1-octanol into primary amines in the presence of ammonia. Our results suggest that ruthenium nanoparticle size is one of the most important parameters, which affects the catalytic performance of titania supported catalysts in the amination reactions. The selectivity to primary amines is much higher over smaller supported Ru nanoparticles than over larger ones, especially in the liquid-phase amination of 1-octanol. The 95% selectivity to octylamine is obtained over small supported Ru nanoparticles with a diameter of 1.4 nm even at 95% conversion, whereas for larger Ru nanoparticles, the octylamine selectivity drops as the 1-octanol conversion approached 80–90%. The drop of the selectivity to octylamine at higher conversion is due to the increase in the intrinsic rate of octylamine coupling over larger ruthenium nanoparticles. The support can also contribute to some extent to the rate of primary amine self-coupling, while its effect on the overall catalytic performance is not significant for the titania-supported catalysts.


 Please wait while we load your content...
Please wait while we load your content...