3DOM CeO2-supported RuyM (M = Au, Pd, Pt) alloy nanoparticles with improved catalytic activity and chlorine-tolerance in trichloroethylene oxidation†
Abstract
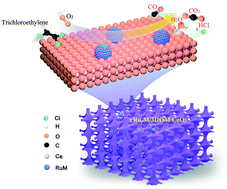
Ruthenium-based materials are promising catalysts for the removal of chlorine-containing volatile organic compounds (CVOCs) due to their good ability in cleaving the C–Cl bonds and decreasing the Cl deposition. However, the roles of noble bimetals alloyed with Ru have not been clarified clearly. In this work, the bimetallic RuyM (M = Au, Pd or Pt) alloys supported on three-dimensionally ordered macroporous (3DOM) CeO2 (xRuyM/3DOM CeO2; RuyM loading (x) = 0.90–0.93 wt%, and Ru/M molar ratio (y) = 2.77–2.87) were fabricated by the polymer microbead-templating and polyvinyl alcohol-protected reduction methods, and their physicochemical properties were well measured using a number of techniques. Oxidation of trichloroethylene (TCE) was used to evaluate catalytic performance of the as-obtained samples with a good-quality 3DOM structure and a surface area of 39–44 m2 g−1. The Ru, Pd, and RuyM nanoparticles (NPs) with an average size of 2.9–3.5 nm were uniformly dispersed on the skeleton surface of 3DOM CeO2. The 0.93Ru2.87Pd/3DOM CeO2 sample showed the highest catalytic activity and the lowest apparent activation energy (34 kJ mol−1), with the temperatures (T50% and T90%) at TCE conversions of 50 and 90% being 237 and 298 °C at a space velocity of 20 000 mL g−1 h−1, respectively. Simultaneously, 0.93Ru2.87Pd/3DOM CeO2 exhibited the highest TCE oxidation rate (30.3 μmol gNoblemetal−1 s−1) and the highest TOFNoblemetal (3.1 × 10−3 s−1) at 250 °C. The 0.93Ru2.87Pd/3DOM CeO2 sample also possessed a better hydrothermal stability than the 0.85Ru/3DOM CeO2 sample after hydrothermal ageing treatment at 750 °C. Effects of H2O, CO2, and HCl on catalytic activity of 0.93Ru2.87Pd/3DOM CeO2 were also examined. The presence of Ru in the samples was favorable for generation of HCl and Cl2 and reduction of the by-products in TCE oxidation, and tetrachloroethylene was the main by-product. The possible catalytic mechanism over 0.93Ru2.87Pd/3DOM CeO2 was also proposed. The outstanding catalytic efficiency of 0.93Ru2.87Pd/3DOM CeO2 could be assigned to the high adsorbed oxygen species, good low-temperature reducibility, strong TCE adsorption and activation ability, and formation of the intimate nanointerface between RuPd NPs and 3DOM CeO2. This work provides a practical guideline for rationally designing the efficient bimetallic catalysts for CVOCs removal under the industrial conditions.


 Please wait while we load your content...
Please wait while we load your content...