Tuning the reactivity of ethylene oligomerization by HZSM-5 framework Alf proximity†
Abstract
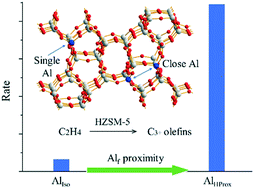
Ethylene oligomerization with controlled chain lengthening over the shape-selective HZSM-5 catalyst provides alternative technologies for the production of C3+ light olefins under the current circumstances of shifting hydrocarbon feedstocks from naphtha to ethane. Here, by tuning the MFI framework Alf site to either isolated or in close proximity, we investigated the influence of the Alf proximity on the kinetics and mechanisms of ethylene oligomerization. We demonstrate that the HZSM-5 catalyst with a high concentration of Alf in close proximity (AlHProx) shows higher activity. The reaction rate of AlHProx is up to 14-fold that of AlIso (HZSM-5 with isolated Alf). Steady-state kinetic studies showed that the activation energy for AlIso (104.8 kJ mol−1) was lower than that for AlHProx (144.5 kJ mol−1). In addition, the kinetic compensation effect with the isokinetic point (Tiso) at 177 °C and Rateiso at 0.015 μmol g−1 s−1 was identified. Further reaction transient analysis suggested that the dual-cycle hydrocarbon-pool (HP) mechanism was involved. Unlike the steady state, the transient state favors the formation of alkanes and aromatics, indicating the establishment of cyclic carbocations through oligomerization, cyclization, and hydride transfer. The early-stage (HP build-up) behavior seemed to be independent of the Alf proximity, but the back-transient (HP clean-up) suggested decrease in the aromatic cycle over the AlHProx sample.


 Please wait while we load your content...
Please wait while we load your content...