One-pot, chemoselective synthesis of secondary amines from aryl nitriles using a PdPt–Fe3O4 nanoparticle catalyst†
Abstract
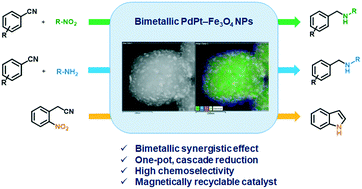
We have developed a new catalytic method for the one-pot, cascade synthesis of unsymmetrical secondary amines via the reductive amination of aryl nitriles with nitroalkanes using a PdPt–Fe3O4 nanoparticle (NP) catalyst. The use of a bimetallic catalyst resulted in enhanced reactivity and selectivity compared to that of either monometallic Pd–Fe3O4 or the Pt–Fe3O4 NP catalyst. Using this bimetallic catalytic system, we were successful in the synthesis of various unsymmetrical secondary amines under mild conditions. However, aryl nitriles containing an electron-donating substituent were rather resistant to the reductive amination, and when hexafluoroisopropanol (HFIP) was used as a co-solvent, the reaction selectivity and yield for unsymmetrical secondary amines increased dramatically. Using the catalyst system, one-pot, gram-scale synthesis of indole was possible from 2-nitrophenylacetonitrile. Due to the magnetic property of the Fe3O4 support, the bimetallic catalyst could easily be recycled using an external magnet at least four times.


 Please wait while we load your content...
Please wait while we load your content...