Coking-resistant dry reforming of methane over BN–nanoceria interface-confined Ni catalysts†
Abstract
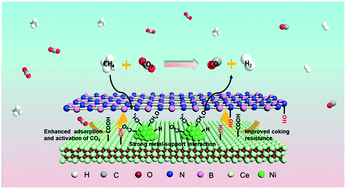
Dry reforming of methane (DRM) over Ni-based catalysts is a promising method to convert greenhouse gases CH4 and CO2 into valuable synthesis gas. However, the major drawback of DRM over Ni-based catalysts is its deactivation due to metal sintering and coke formation. Herein, Ni catalysts confined between boron nitride (BN)–nanoceria (NC) interfaces have been originally developed and demonstrated as efficient and stable DRM catalysts, which exhibit high activity and high resistance towards carbon deposition. The stronger interaction between nickel and BN–nanoceria interfaces led to higher concentration of Ce3+ species, which promotes adsorption and activation of CO2 and thus facilitates the fast formation of –OH species. The active –OH species is crucial to prevent coke formation and improve the stability of Ni catalysts. This work demonstrates that restriction of metal nanoparticles within a nano-interface through the confinement effect is a successful strategy to develop coke-resistant catalysts.


 Please wait while we load your content...
Please wait while we load your content...