Synthesis of a vinyl chloride monomer via acetylene hydrochlorination with a ruthenium-based N-heterocyclic carbene complex catalyst†
Abstract
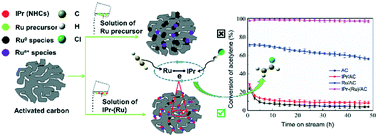
With the implementation of the Minamata Convention and the urgent demand for green and efficient mercury-free catalysts, a carbon-supported IPr–(Ru)/AC catalyst was synthesized and assessed for its successful application in acetylene hydrochlorination. The results showed that the interplay between the central Ru and surrounding IPr ensured the stability and high-level dispersion of the active species on the surface of the host; the accelerated electron transfer from the N-heterocycle to Run+ boosted the electron cloud density around the active centres, increasing the relative amount of the active constituents. The delocalisation and transfer of electrons in IPr–(Ru) might synergistically improve the ability of the IPr- and Ru-sections in the catalyst to adsorb and activate H–Cl and C2H2, respectively, thus yielding a significant improvement in the original catalytic activity with respect to its counterpart. In addition, the complexation also greatly slowed down the deactivation of the catalyst and endowed the catalyst with a potential industrial application combined with its impressive stability with respect to the original Ru/AC.


 Please wait while we load your content...
Please wait while we load your content...