Deoxydehydration of 1,4-anhydroerythritol over anatase TiO2(101)-supported ReOx and MoOx†
Abstract
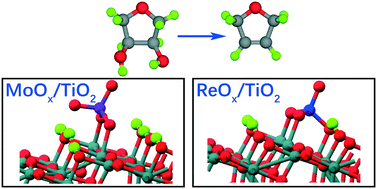
Heterogeneously catalyzed deoxydehydration (DODH) ordinarily occurs over relatively costly oxide supported ReOx sites and is an effective process for the removal of vicinal OH groups that are common in biomass-derived chemicals. Here, through first-principles calculations, we investigate the DODH of 1,4-anhydroerythritol over anatase TiO2(101)-supported ReOx and MoOx. The atomistic structures of ReOx and MoOx under typical reaction conditions were identified with constrained thermodynamics calculations as ReO2(2O)/6H–TiO2 and MoO(2O)/3H–TiO2, respectively. The calculated energy profile and developed microkinetic reaction model suggest that both ReO2(2O)/6H–TiO2 and MoO(2O)/3H–TiO2 exhibit a relatively low DODH activity at 413 K. However, at higher temperatures such as 473 K, MoO(2O)/TiO2(101) was found to exhibit a reasonably high catalytic activity similar to ReO2(2O)/6H–TiO2, consistent with a recent experimental study. Mechanistically, the first O–H bond cleavage of 1,4-anhydroerythritol and the dihydrofuran extrusion were found to be the rate-controlling steps for the reaction over ReO2(2O)/6H–TiO2 and MoO(2O)/3H–TiO2, respectively. Thus, this study clarifies the mechanism of the DODH over oxide-supported catalysts and provides meaningful insight into the design of low-cost DODH catalysts.


 Please wait while we load your content...
Please wait while we load your content...