Enhanced reduction and oxidation capability over the CeO2/g-C3N4 hybrid through surface carboxylation: performance and mechanism†
Abstract
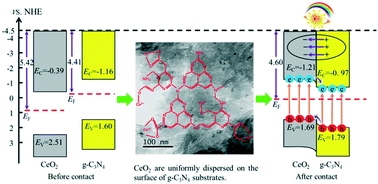
The CeO2/g-C3N4 hybrid is a kind of efficient photocatalyst with both photoinduced oxidation and reduction capability, which is of great concern in solar energy application. Herein, we reported a facile method for the synthesis of the CeO2/g-C3N4 hybrid with enhanced CO2 reduction and ciprofloxacin degradation performance using surface carboxylated g-C3N4 (C-g-C3N4) as the substrate. The characterization results demonstrated the abundant oxygen-containing groups of C-g-C3N4 in the CeO2/C-g-C3N4 hybrid can effectively improve the dispersion of CeO2 nanoparticles and enhance the interfacial bonding with the C-g-C3N4 substrate. Density functional theory (DFT) calculations showed that a built-in electric field was formed in the CeO2/C-g-C3N4 heterojunction, which can greatly improve the charge separation and transfer efficiency. Consequently, the yield of CO and the ciprofloxacin degradation efficiency have been remarkably improved. The maximum CO yield through CO2 photoreduction over 3%CeO2/C-g-C3N4 was 9.083, 3.922, and 2.868 times higher than that of pure CeO2, C-g-C3N4 and 3%CeO2/g-C3N4 bulk, respectively. The 3%CeO2/C-g-C3N4 heterojunction also showed excellent photoinduced oxidation activity for ciprofloxacin degradation with a 73% degradation efficiency in 2 h, which was 1.89 and 2.76 times higher than that of pure CeO2 and C-g-C3N4, respectively. Furthermore, a good photostability for a five cycle test of CO2 reduction was observed over the 3%CeO2/C-g-C3N4 hybrid. The possible photocatalytic mechanism was investigated by theoretical calculations and capture experiments to further understand the charge transfer behavior over the CeO2/C-g-C3N4 heterojunction for CO2 reduction and pollutant degradation.


 Please wait while we load your content...
Please wait while we load your content...