Highly selective hydrogenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran at low temperature over a Co–N–C/NiAl-MMO catalyst†
Abstract
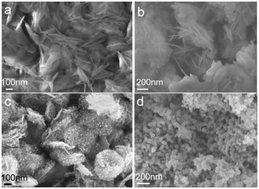
Currently there is tremendous interest in the discovery of catalysts which can selectively hydrogenate biomass-derived 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF). Herein, a highly selective catalyst for this transformation was developed, by adsorption of a cobalt porphyrin (CoTAPP) onto a nickel–aluminium layered double hydroxide (NiAl-LDH) support, followed by a pyrolysis step at 500 °C under a N2 atmosphere. The obtained catalyst (denoted here as Co–N–C/NiAl-MMO), comprising cobalt species (Co0 and CoOx) and N-doped carbon on a NiAl mixed metal oxide support, showed outstanding initial selectivity (99.9%) for the hydrogenation of HMF to DMF at 170 °C in tetrahydrofuran (THF). This is one of the highest selectivities reported to date for this reaction, with the reaction temperature being very mild. After 3 cycles of catalytic tests, with catalyst regeneration by heating at 300 °C in N2 between tests, the HMF conversion efficiency and DMF selectivity of Co–N–C/NiAl-MMO had both decreased by >70% compared to the initial values. This deactivation resulted from the loss of surface basic sites needed for H2 activation, as well as a change in the Co speciation on the catalyst surface (i.e. Co0 oxidation to CoOx). Results guide the development of improved catalysts for the selective conversion of HMF to DMF.


 Please wait while we load your content...
Please wait while we load your content...