Stability of the ketyl radical as a descriptor in the electrochemical coupling of benzaldehyde†
Abstract
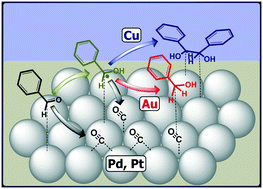
Electroreductive coupling is an emerging electrochemical pathway for the renewable upgrading of CO2, CO and biomass derived oxygenates. In particular, electrochemical coupling of molecules with a carbonyl group has the potential to grow the molecular weight of substrates via C–C bond formation to produce valuable fuels and chemicals. However, a lack of mechanistic understanding hinders rational catalyst development for this coupling chemistry. In this work, the electrochemical reduction of benzaldehyde is employed as a model reaction to investigate the impact of four metals (Au, Cu, Pd and Pt) on the two carbonyl reduction pathways, i.e., direct electrochemical reduction to benzyl alcohol and reductive C–C coupling to a diol product, i.e., hydrobenzoin. Reactivity studies show that, of the metals tested, Cu has a unique ability to mediate the C–C coupling of benzaldehyde. Complementary in situ spectroscopic investigations suggest that this facilitation of C–C coupling is directly related to the ability of the Cu catalyst to stabilize a key reaction intermediate, i.e., the ketyl radical. Spectroscopic features of the ketyl radical are observed on Au and Cu surfaces at benzaldehyde reduction potentials, but not on Pd and Pt. A lower radical concentration for Au compared to Cu is likely the primary reason for the lack of C–C coupling on Au. On the Pd and Pt surfaces, CO formation is observed from dissociative adsorption and decarbonylation upon benzaldehyde introduction, suggesting surface adsorbate instability under reducing conditions. From the combined reactivity and spectroscopic evidence, we propose that the ability of a catalyst to produce and stabilize the ketyl radical intermediate is a key descriptor in its ability to mediate the C–C coupling chemistry of aldehydes and ketones.


 Please wait while we load your content...
Please wait while we load your content...