Importance of the synergistic effects between cobalt sulfate and tetrahydrofuran for selective production of 5-hydroxymethylfurfural from carbohydrates†
Abstract
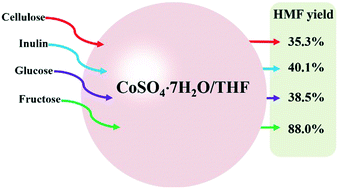
In this study, an effective catalytic system (CoSO4·7H2O/THF) for selective conversion of fructose to 5-hydroxymethylfurfural (HMF; yield: 88%) was developed. The synergistic effects among Co2+, SO42−, crystal water and tetrahydrofuran (THF) were crucial for achieving selective dehydration of fructose to HMF. Co2+ worked as a Lewis acid for catalyzing mainly dehydration of fructose to HMF but not the further decomposition of HMF to levulinic acid. THF could help to retain HMF while CoSO4 could coordinate with HMF, enhancing the thermal stability of HMF in THF. The crystal water in cobalt sulfate could help to coordinate with fructose, which facilitated the conversion of fructose via dehydration reactions. The CoSO4·7H2O/THF catalytic system could also catalyze the conversion of inulin and cellulose into HMF. The main advantages of the CoSO4·7H2O/THF catalytic system are the low cost, the easy recycling of the CoSO4·7H2O catalyst and the easy separation of HMF from volatile THF.


 Please wait while we load your content...
Please wait while we load your content...