Porous manganese oxides synthesized with natural products at room temperature: a superior humidity-tolerant catalyst for ozone decomposition†
Abstract
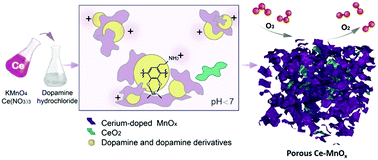
Ozone is a prevalent pollutant threatening human health worldwide; hence, the search for efficient humidity-tolerant catalysts for ozone removal is of great importance. In this study, porous cerium-doped manganese oxides (Ce–MnOx) with superior humidity-tolerant activity for ozone decomposition were synthesized via a 1 hour reaction between permanganate, dopamine and Ce3+ at room temperature without any thermal treatment. Herein, dopamine, which is widely distributed in nature, acts as an easy reductant and also inhibits the growth and crystallization of manganese oxides (MnOx) via in situ coordination. The addition of Ce3+ further inhibits the growth of MnOx and gives rise to more acidic solutions, which facilitates the dispersion of the MnOx nucleus coordinated with the protonated dopamine derivatives. Consequently, porous Ce–MnOx with abundant macropores and mesopores was obtained, which consists mostly of amorphous MnOx; prevalent atomic doping and embedded nanocrystals were revealed by HAADF-STEM. The as-synthesized hierarchical porous Ce–MnOx exhibited excellent activity and humidity tolerance for O3 decomposition, achieving 100% decomposition of 100 ppm O3 within the 10 h test under a high space velocity of 540 L gcat−1 h−1 at 50% RH and 25 °C.


 Please wait while we load your content...
Please wait while we load your content...