Enhanced oxygen evolution catalysis by aluminium-doped cobalt phosphide through in situ surface area increase†
Abstract
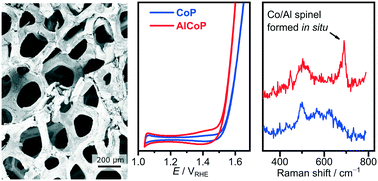
The deployment of water electrolysis as a major contributor to global hydrogen production requires the elimination of catalysts based on scarce and expensive precious metals, and amongst the most promising alternatives are first-row transition metal phosphides. This study presents the synthesis, characterisation, electrochemical testing and performance rationalisation of cobalt phosphide modified with aluminium as an improved catalyst for alkaline oxygen evolution. The electrodes were prepared by gas phase phosphorisation of Al-sputtered Co foam, and characterised by SEM, EDX, XRD, XPS, HAADF-STEM and Raman spectroscopy. Al modification enhances the oxygen evolution performance of the anodes, with a current density of 200 mA cm−2 reached at an overpotential of 360 mV, representing a 50 mV improvement compared to the Al-free sample. Double layer capacitance measurements indicate that the performance enhancement results from an approximately four-fold increase in relative electrochemically active surface area (ECSA) in the Al-modified sample. In situ Raman spectroscopy rationalises this ECSA increase on the grounds of an Al-induced preference for a spinel phase Co/Al oxide on the catalyst surface upon exposure to electrolyte solution, the compact crystal structure of which causes shrinkage and surface cracking. This contrasts with previous observations on Al-doped nickel phosphides, where an increase in surface area was attributed to Al dissolution. These results present a route for achieving high current density oxygen evolution without the need to alter the catalyst active species, as well as demonstrate the importance of in situ techniques for rationalising performance improvements resulting from subtle differences in surface chemistry.


 Please wait while we load your content...
Please wait while we load your content...