From CO or CO2?: space-resolved insights into high-pressure CO2 hydrogenation to methanol over Cu/ZnO/Al2O3†
Abstract
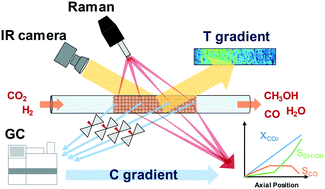
The reaction pathway of high-pressure CO2 hydrogenation over a Cu/ZnO/Al2O3 catalyst is investigated through the gradients of reactants/products concentration and catalyst temperature within the catalytic reactor. This study reveals that methanol is formed through direct CO2 hydrogenation at low temperature, while above 260 °C methanol formation is mediated via CO which is formed by reverse water–gas shift reaction.


 Please wait while we load your content...
Please wait while we load your content...