Effects of carbon number and bond saturation on hydrocarbon combustion over a diesel oxidation catalyst
Abstract
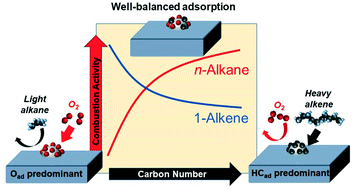
One of the problems in a diesel oxidation catalyst is its insufficient activity for injected diesel fuel at low temperatures caused by strong adsorption of hydrocarbons. This study systematically clarified the poisoning effect of various hydrocarbons having carbon numbers from 3 to 16. As the carbon number in alkanes increased, the light-off temperature over a Pt–Pd/Al2O3 catalyst gradually shifted to a lower temperature. While alkenes and aromatics showed an opposite trend, the light-off shifted to a higher temperature as the carbon number increased. In situ FTIR spectra indicated that the amount of adsorbed hydrocarbons on the metal surface increased with the increase in the carbon number of alkanes and alkenes. Kinetic analysis showed that the reaction orders with respect to O2 were below zero in light alkane combustion, while those with respect to hydrocarbons were zero or lower in alkene and aromatic combustion. The shift of the light-off temperatures was rationalized by competitive adsorption of hydrocarbons and oxygen.


 Please wait while we load your content...
Please wait while we load your content...