Chiral phosphoric acid catalyzed asymmetric arylation of indoles via nucleophilic aromatic substitution: mechanisms and origin of enantioselectivity†
Abstract
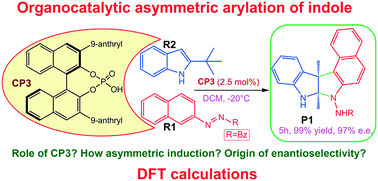
Asymmetric arylation of indole via a nucleophilic aromatic substitution reaction can be effectively realized when chiral phosphoric acid serves as a catalyst in the reaction between azobenzene derivative and indole, producing chiral pyrroloindolines with high yields and excellent enantioselectivity. To elaborate on the reaction processes and origin of enantioselectivity, quantum mechanical calculations were carried out. Both global and local reactivity indexes were employed to understand the reactivity of substrates. The calculations show that eight pathways exist that lead to the products. It was found that chiral phosphoric acid interacts with the substrates via dual hydrogen-bonding activation modes, and it can tautomerize itself to realize both proton and electron transfer and thus promote C–C and C–N bond formation. Quantum theory of atoms in molecules and noncovalent interaction analyses were used to illustrate the roles of weak intermolecular interactions. The origin of enantioselectivity was illustrated by analyzing the stability of the complex and transition state. The calculated e.e. value was 92%, which is in accordance with the experimental value. We expect the results will be insightful for understanding asymmetric reactions catalyzed by chiral phosphoric acid.


 Please wait while we load your content...
Please wait while we load your content...