The competition between dehydrogenation and dehydration reactions for primary and secondary alcohols over gallia: unravelling the effects of molecular and electronic structure via a two-pronged theoretical/experimental approach†
Abstract
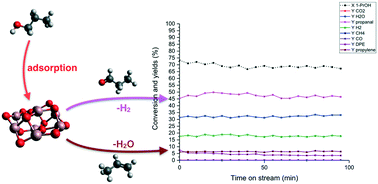
To explore alternative applications of gallium(III) oxide and co-precipitated Ga(III)/M(II) oxides (M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mg, Ca, and Sr), we investigate the relative dehydrogenation/dehydration activity of nanostructured gallia for alcohols via electronic structure calculations, reactivity tests and DRIFT-IR spectroscopy. Computing the relative reaction barriers with all electron DFT and split-valence basis sets for a panel of 11 alcohols suggested dehydrogenation to be the most active process catalysed by gallia for both primary and secondary alcohols despite its fundamentally acidic behaviour, which was previously suggested to foster dehydration. A key contribution in defining the most active channel is also provided by the interaction between the alcohol carbon skeleton and gallia surface. The only deviation from the relative reactivity just discussed is found for 1-phenyl ethanol, an outcome easily rationalized by the conjugated nature of the nearly-carbocationic TS leading to styrene. Catalytic tests on methanol, ethanol, and 1- and 2-propanol at 400 °C supported the proposed relative selectivity; besides, the recorded alcohol total conversions and product yields agree well with what was proposed by the DFT reaction profiles, thus demonstrating the predictive capability of the chosen electronic structure approach. Finally, temperature programmed DRIFT spectroscopy on the aliphatic C3 alcohols was able to characterize the adsorbed reactants, intermediates, and products, fully supporting the picture described by the theoretical modelling and the reactivity tests. In conclusion, the lower acidity of Ga(III), due to its semi-metallic nature and its larger ionic radius, compared to that of Al(III), leads to opposite selectivity in the competition between alcohol dehydrogenation and dehydration at 400 °C, with the oxide of the latter ion being known to preferentially produce olefins.
Mg, Ca, and Sr), we investigate the relative dehydrogenation/dehydration activity of nanostructured gallia for alcohols via electronic structure calculations, reactivity tests and DRIFT-IR spectroscopy. Computing the relative reaction barriers with all electron DFT and split-valence basis sets for a panel of 11 alcohols suggested dehydrogenation to be the most active process catalysed by gallia for both primary and secondary alcohols despite its fundamentally acidic behaviour, which was previously suggested to foster dehydration. A key contribution in defining the most active channel is also provided by the interaction between the alcohol carbon skeleton and gallia surface. The only deviation from the relative reactivity just discussed is found for 1-phenyl ethanol, an outcome easily rationalized by the conjugated nature of the nearly-carbocationic TS leading to styrene. Catalytic tests on methanol, ethanol, and 1- and 2-propanol at 400 °C supported the proposed relative selectivity; besides, the recorded alcohol total conversions and product yields agree well with what was proposed by the DFT reaction profiles, thus demonstrating the predictive capability of the chosen electronic structure approach. Finally, temperature programmed DRIFT spectroscopy on the aliphatic C3 alcohols was able to characterize the adsorbed reactants, intermediates, and products, fully supporting the picture described by the theoretical modelling and the reactivity tests. In conclusion, the lower acidity of Ga(III), due to its semi-metallic nature and its larger ionic radius, compared to that of Al(III), leads to opposite selectivity in the competition between alcohol dehydrogenation and dehydration at 400 °C, with the oxide of the latter ion being known to preferentially produce olefins.


 Please wait while we load your content...
Please wait while we load your content...