New findings and current controversies in the reaction of ruthenium red and ammonium cerium(iv) nitrate: focus on the precipitated compound†
Abstract
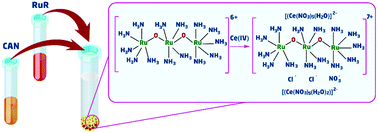
Finding the true catalyst has always been a challenging step toward the exploration, understanding, and design of catalytic systems for water-oxidation reaction. Many homogeneous and heterogeneous transition metal complexes have been reported that can undergo a complete transformation to the true catalysts during water-oxidation reaction. Herein, the transformation of the previously-explored ruthenium red ([(NH3)5RuORu(NH3)4ORu(NH3)5]Cl6) catalyst in the presence of ammonium cerium(IV) nitrate is reported. For the first time, it is reported that a heterogeneous nano-sized ruthenium–cerium compound is formed by the electrostatic interaction of [(NH3)5RuORu(NH3)4ORu(NH3)5],6+/7+ nitrate ions, and the products of the reduction of ammonium cerium(IV) nitrate.


 Please wait while we load your content...
Please wait while we load your content...