Hydrogen bonding and non-covalent interaction assisted nickel(0) catalysed reversible alkenyl functional group swapping: a computational study†
Abstract
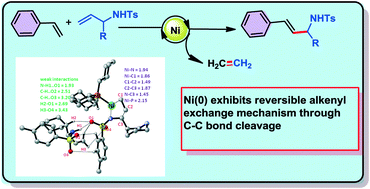
An extensive DFT study is carried out to explore the mechanistic pathways involved in nickel(0) catalysed functional group swapping, where the less substituted N-tosyl allylamine transforms into a more substituted N-tosyl allylamine product. Here, we demonstrated the active nickel catalyst species and transformative reaction intermediates involved in the product formation under given reaction conditions. The H-bonding and non-covalent interactions offered by a second molecule of N-tosyl allylamine coordinated to the nickel centre play a crucial role in stabilizing the key intermediates and the transition states involved in the C(2)–C(3) bond cleavage pathway. In this reversible alkenyl exchange reaction, the C(2)–C(3) bond cleavage pathway proceeds through aza-nickelacyclopentane.


 Please wait while we load your content...
Please wait while we load your content...