Micro-kinetic modelling of photocatalytic CO2 reduction over undoped and N-doped TiO2†
Abstract
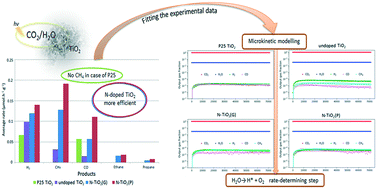
CO2 photoreduction is studied using in-house synthesized undoped and N-doped TiO2 nanoparticle photocatalysts. Comparison with the commercial P25 TiO2 powder shows that the synthesized samples are more effective. P25 produced a negligible amount of CH4, unlike the synthesized samples. N-doping of TiO2 powder caused a higher productivity rate of all products, and provided the best performance for CO2 reduction. The average production rate of CH4 was 0.191 μmol h−1 g−1, whereas that of CO was 0.111 μmol h−1 g−1. The experimental data are used to fit the micro-kinetic modelling parameters. The kinetic constant of H2O dissociation is the lowest for all tested samples, revealing that this is the rate-determining step. The kinetic constants for the H2, CO, and CH4 formation were of the same order for all catalyst samples, showing that the rates of these reactions are independent of the catalyst type.
- This article is part of the themed collection: 2020 Catalysis Science & Technology Hot Articles


 Please wait while we load your content...
Please wait while we load your content...