Carbon nanotube-supported Cu-based catalysts for oxidative carbonylation of methanol to methyl carbonate: effect of nanotube pore size
Abstract
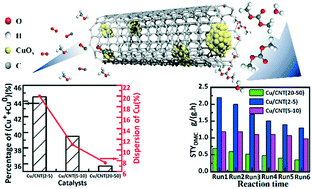
Carbon nanotubes (CNTs) with three different inner diameters (2–5 nm, 5–10 nm and 20–50 nm) were employed as supports to prepare Cu-based catalysts for oxidative carbonylation of methanol to dimethyl carbonate (DMC). The catalysts were extensively characterized by N2-physisorption, TEM, H2-TPR, XRD, TPD-MS, in situ-XRD, XPS, N2O chemisorption and AAS. The results indicated that CNTs with a small inner diameter were favorable for the entry of Cu species into the cavity and the formation of highly dispersed Cu particles. The highly dispersed Cu species presented strong interaction with CNTs, which facilitated the auto-reduction of CuO to active Cu2O and Cu during the calcination. The dispersion and percentage of active Cu species for Cu/CNT (2–5) were maximum among all the catalysts, and hence it presented the optimal initial activity for oxidative carbonylation of methanol. The conversion of methanol and the space time yield of DMC were 4.2% and 2.2 g g−1 h−1), respectively. Moreover, the stability of Cu species was closely related to their particle size, the inner diameter of CNTs and the interaction between Cu species and CNTs. The Cu species of Cu/CNT (20–50) seriously aggregated after the reaction due to the weak interaction between Cu species and CNT (20–50) as well as the insufficient confinement effect of CNT (20–50). CNT (2–5) presented the strongest interaction with Cu species; however, it cannot effectively retard the migration and agglomeration of Cu species resulting from the extremely high surface energy of these small particles (1–3 nm). Unlike Cu/CNT (2–5) and Cu/CNT (20–50), the particles of Cu species for Cu/CNT (5–10) were more stable owing to the modest particle size and the moderate interaction between Cu species and CNT (5–10). Furthermore, the enhanced interaction between Cu species and CNTs can effectively suppress the oxidation of Cu species during the reaction. The deactivation of Cu/CNT (20–50) was due to the agglomeration and oxidation of Cu species, and the deactivation of Cu/CNT (2–5) was due to the agglomeration of Cu species, whereas that of Cu/CNT (5–10) was due to the oxidation of Cu species. Cu/CNT (5–10) exhibited the optimal stability.


 Please wait while we load your content...
Please wait while we load your content...