Effect of ceria morphology on the performance of MnOx/CeO2 catalysts in catalytic combustion of N,N-dimethylformamide†
Abstract
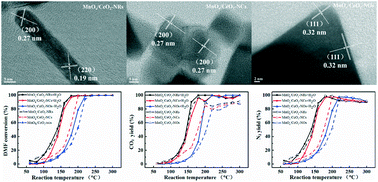
MnOx/CeO2 catalysts were prepared by a deposition–precipitation method, through loading MnOx into ceria supports with different morphologies (nanorods (NRs), nanocubes (NCs) and nano-octahedrons (NOs)). As for the catalytic combustion of N,N-dimethylformamide (DMF), the ceria morphology has a great impact on the catalytic activity, and loading of MnOx significantly enhances the catalytic activity as follows: MnOx/CeO2-NRs > MnOx/CeO2-NCs > MnOx/CeO2-NOs > CeO2-NRs > CeO2-NCs > CeO2-NOs. The MnOx/CeO2-NR catalyst exhibits the best catalytic performance, in which DMF is completely oxidized at 180 °C, and the CO2 and N2 yields are 98% and 99%, respectively. The catalytic performance of MnOx/CeO2 catalysts at high reaction temperature under the wet reaction conditions with introduction of 5 vol% water vapor is better than that under the dry reaction conditions, in which water easily desorbs from the catalyst surface and then reacts with the reaction intermediates. The results of nitrogen sorption, XRD, O2-TPD, XPS and NH3-TPD characterization indicate that the excellent catalytic performance of the MnOx/CeO2-NR catalyst is closely related to its high specific surface area, high concentration of oxygen vacancies and active oxygen species, and more and stronger surface acid sites, which are due to the strong interaction between MnOx and CeO2 nanorods.


 Please wait while we load your content...
Please wait while we load your content...