Quantitative determination of the Cu species, acid sites and NH3-SCR mechanism on Cu-SSZ-13 and H-SSZ-13 at low temperatures†
Abstract
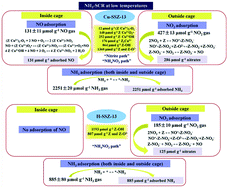
The adsorption amounts of NO, NO2, and NH3, the number of adsorption sites including different Cu species and acid sites and the reaction mechanism of NH3-SCR on Cu-SSZ-13 synthesized by a one-pot synthesis method and H-SSZ-13 at low temperatures were determined in the present study by a quantitative analysis method, the transient response method (TRM). NH3 covered most of the surface sites on both Cu-SSZ-13 and H-SSZ-13 during the NH3-SCR reaction. NO2 adsorbed on the SSZ-13 zeolite support, and NO adsorbed on Cu sites. The presence of O2 promoted NO adsorption on Cu sites. However, H-SSZ-13 could not activate NO either in the presence or absence of O2. Cu-SSZ-13 was composed of 12 μmol g−1 (Z−Cu2+)2–O2, 148 μmol g−1 Z−Cu2+O2˙ and 252 μmol g−1 Z−Cu2+OH for NO and NH3 adsorption, 176 μmol g−1 Z2Cu2+ and 864 μmol g−1 Z–OH for NH3 adsorption, and 1360 μmol g−1 Z and Z–O2− for NO2 adsorption, while H-SSZ-13 consisted of 1193 μmol g−1 Z–OH for NH3 adsorption and 807 μmol g−1 Z and Z–O2− for NO2 adsorption. On H-SSZ-13, two NH3 molecules adsorbed on one acid site, and the only reaction pathway was NO reacting with NH4NO3 to form N2, H2O and NO2 (“NH4NO3 pathway”) during standard and fast SCR. On Cu-SSZ-13, besides the “NH4NO3 pathway” on the SSZ-13 support, reactions between nitrite formed by the NO adsorbed on Z−Cu2+–O2˙, (Z−Cu2+)2–O2 and Z−Cu2+OH and adsorbed NH3 to form N2 and H2O (“nitrite pathway”) also occurred. Cu-SSZ-13 exhibited higher NH3-SCR activity than H-SSZ-13 because of the reaction between adsorbed NH3 and nitrite formed by NO adsorbed on the Cu sites in the CHA cages.


 Please wait while we load your content...
Please wait while we load your content...