Amorphous nickel–iron hydroxide films on nickel sulfide nanoparticles for the oxygen evolution reaction†
Abstract
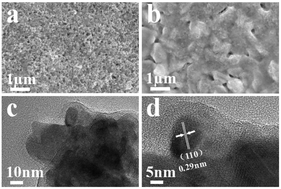
The development of earth-abundant and low-cost electrocatalysts with high performance toward the oxygen evolution reaction (OER) plays a key role in water splitting. Here, a novel OER electrocatalyst of Ni–Fe–OH/Ni3S2 nanoparticles on Ni foam (Ni–Fe–OH/Ni3S2/NF) was synthesized by a facile, ultrafast two-step method within 2 minutes. The obtained Ni–Fe–OH/Ni3S2/NF catalyst presents an excellent OER performance, only requiring a low overpotential of 268 mV to reach a current density of 10 mA cm−2 and a small Tafel slope of 54 mV dec−1 in 1 M KOH, which was sustained for 12 h at a current density of 175 mA cm−2 almost without any degradation. The superior OER performance of the Ni–Fe–OH/Ni3S2/NF catalyst is attributed to the increased active sites, the accelerated electron transfer between the electrode and electrolyte, and the synergistic effect between amorphous Ni–Fe–OH and Ni3S2. This work opens up a new avenue for preparing highly efficient heterostructure electrocatalysts toward the OER.
- This article is part of the themed collection: 2020 Catalysis Science & Technology Hot Articles


 Please wait while we load your content...
Please wait while we load your content...