Development of catalysts for ammonia synthesis based on metal phthalocyanine materials†
Abstract
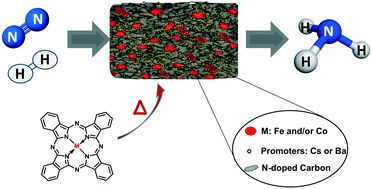
Highly efficient and very stable iron and/or cobalt-based catalysts for the ammonia synthesis reaction were synthesized by one-step pyrolysis of metal phthalocyanine precursors. The presence of alkaline earth or alkali metals is found to be essential for accelerating the reaction rate for the ammonia synthesis process. When promoted by alkali metals, the catalysts show a 3-fold increase in their catalytic performance (at 400 °C and 0.1–7 MPa) compared to a commercial benchmark iron-based catalyst, widely used for the Haber–Bosch process. TEM images reveal the local structure of the catalysts obtained upon pyrolysis of the metal phthalocyanine precursor, with metal nanoparticles (5–50 nm) confined in a nitrogen-doped carbon mesoporous matrix, where the alkali metal promoters are located on the top of the iron nanoparticles but also on the carbon support. Finally, kinetic analysis shows a lower activation energy for the Fe phthalocyanine-derived catalyst (42 kJ mol−1) versus 70 kJ mol−1 reported for the iron-benchmark catalyst. Furthermore, this kinetic analysis suggests that the rate-determining step shifts from nitrogen activation to NHx formation, which only few catalysts have achieved.


 Please wait while we load your content...
Please wait while we load your content...