Effect of catalyst morphology and hydrogen co-feeding on the acid-catalysed transformation of acetone into mesitylene†
Abstract
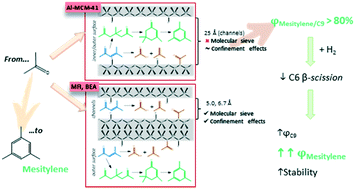
The application of different aluminosilicates (MCM-41, MFI, and BEA) to catalyse the gas-phase self-condensation of acetone allows the selective formation of mesitylene regarding the rest of the C9 compounds (relative selectivity up to 80%). Results suggest that mesitylene formation takes place via isophorones instead of via direct dehydration–cyclization of phorones. However, acetic acid and isobutene are also formed (β-scission of C6 compounds). This side reaction has two negative effects: (i) competition with the target reaction decreasing the mesitylene yield and (ii) formation of intermediate acetate species involved in the acetic acid formation, leading to fast deactivation, especially at initial reaction times. This last point was confirmed by DRIFTS analysis. Co-feeding of molecular hydrogen was considered for improving the catalytic stability and the selectivity to aromatics. In fact, the catalytic activity of MCM-41 and BEA is enhanced, with mesitylene yields around 60 and 100% higher with regard to inert operating conditions, respectively. However, supplying H2 entails a drop in the catalytic dehydration activity due to its interaction with active sites. This fact implies lower relative selectivity to mesitylene regarding all the C9 compounds (ca. 50%). Otherwise, the absolute selectivity to mesitylene is improved since the C6 β-scission reaction is largely hindered by the presence of H2.


 Please wait while we load your content...
Please wait while we load your content...