Prediction of optimal catalysts for a given chemical reaction†
Abstract
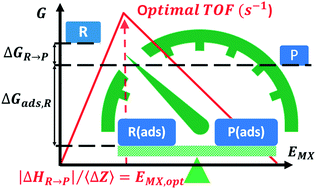
We reveal a correlation between the M–X bond energy descriptor EMX for the optimal catalyst in a family of stoichiometry MiXj and an intensive quantity defined as the standard enthalpy of the catalyzed reaction normalized to one mole of element X transferred by this reaction from reactants to products. M is a transition element, and the stoichiometry MiXj is fixed at the solid/fluid interface by the reaction conditions. We illustrate this for a relevant set of reactions involved in solar energy and industrial applications such as oxygen evolution (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), oxygen reduction (X
O), oxygen reduction (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and hydrogen evolution (X
O) and hydrogen evolution (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H) in electrolysis, hydrodesulfurization of thiophene (X
H) in electrolysis, hydrodesulfurization of thiophene (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), methanation of CO (X
S), methanation of CO (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), hydrogenation of aromatics and alkenes (X
C), hydrogenation of aromatics and alkenes (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), selective oxidation of methane (X
C), selective oxidation of methane (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and ammonia synthesis and decomposition (X
O), and ammonia synthesis and decomposition (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). We propose a quantitative model to explain this unexpected connection: this key finding and its interpretation should accelerate in silico discovery of catalysts.
N). We propose a quantitative model to explain this unexpected connection: this key finding and its interpretation should accelerate in silico discovery of catalysts.
- This article is part of the themed collection: 2020 Catalysis Science & Technology Hot Articles


 Please wait while we load your content...
Please wait while we load your content...