Kinetic study of the methane dry (CO2) reforming reaction over the Ce0.70La0.20Ni0.10O2−δ catalyst
Abstract
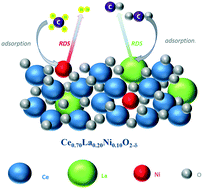
The kinetic behaviour of the Ce0.70La0.20Ni0.10O2−δ catalyst during the methane dry reforming reaction was investigated in a fixed bed reactor in the temperature range of 923–1023 K with the partial pressure of CH4 and CO2 ranging between 5 and 50 kPa. The experimental data were fitted using the empirical power-law rate equation and Langmuir–Hinshelwood kinetic models proposed in literature for the Ni–La2O3 catalytic system and based on two-step single- and dual-site mechanisms. The obtained fitting results, after statistical and thermodynamic discrimination, showed that the mechanism of the dry reforming reaction over the Ce0.70La0.20Ni0.10O2−δ catalyst could be successfully described by the two-step dual-site mechanism. The activation energies for the CH4 consumption from the power law and Langmuir–Hinshelwood models were estimated to be 91.5 and 136.9 kJ mol−1, respectively. The lower activation energies for the CO2 consumption (70.2 and 100.6 kJ mol−1 from both models) suggested that the CO2 activation should faster than CH4. The basic nature of the Ce–La–O sites catalyzed the CO2 conversion to oxy-carbonate, decreasing the CO2 activation energy compared to that of CH4.


 Please wait while we load your content...
Please wait while we load your content...