Effects of SO2 on Cu-SSZ-39 catalyst for the selective catalytic reduction of NOx with NH3†
Abstract
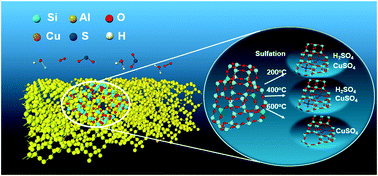
In this work, the Cu-SSZ-39 catalyst was sulfated at different temperatures (200 °C, 400 °C or 600 °C) in the presence of water to investigate the effects of sulfation on its catalytic performance in NH3-SCR. Sulfation at 200 °C and 400 °C resulted in a significant decrease in NOx conversion at low temperatures, while sulfation at 600 °C showed only slight effect on the SCR activity of Cu-SSZ-39. The coverage of active sites by H2SO4 was the main reason for the deactivation of the catalyst sulfated at 200 °C and 400 °C, while the formation of stable CuSO4 species should also be responsible for the catalytic activity loss of the catalysts sulfated at 200 °C, 400 °C and 600 °C. After regeneration at 600 °C, the activities of the sulfated catalysts could be recovered partially but were still lower than those of the fresh catalysts, due to the formation of stable sulfated Cu species.


 Please wait while we load your content...
Please wait while we load your content...