Regeneration of water-deactivated Cu/SAPO-34(MO) with acids†
Abstract
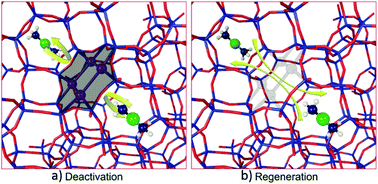
The Cu/SAPO-34 catalysts, used for NH3 selective catalytic reduction (SCR), are systemically studied with various characterization techniques before and after low temperature water deactivation and regeneration using techniques such as XRD, BET, ICP-SFMS, 27Al MAS NMR, 29Si MAS NMR, and H2-TPR. Analysis of results suggests that, during low-temperature water deactivation, hydrolysis of Si–O–Al occurs resulting in Si condensation and formation of Si clusters. It is proposed that these formed Si clusters are mainly responsible for the deactivation of Cu/SAPO-34 catalysts since they suppress the mobility of [Cu–(NH3)]+ and hinder the formation of the transient [CuI(NH3)2]+–O2–[CuI(NH3)2]+ intermediate, which is considered to be the rate-limiting step for NH3-SCR reaction. The regeneration of the deactivated Cu/SAPO-34 catalysts with acid can be explained by the ability of the acid to convert the Si clusters back to Si–O–H, which is able to revert to the SAPO-34 framework via reverse hydrolysis as the temperature increases.


 Please wait while we load your content...
Please wait while we load your content...