Mechanistic insights into Cu-catalyzed enantioselective Friedel–Crafts reaction between indoles and 2-aryl-N-sulfonylaziridines†
Abstract
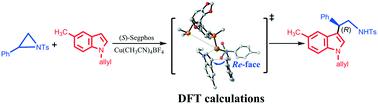
Computational studies were successfully carried out to provide mechanistic insights into LCu-catalyzed (L = (S)-Segphos ligand) Friedel–Crafts (F–C) reaction between indoles and 2-aryl-N-sulfonylaziridines. The proposed enantioselective mechanistic roles for the LCu-catalyzed F–C reaction were carefully considered: C–N bond cleavage through a three-membered ring transition state, C–C bond formation, oxidative addition, and reductive elimination. Through the energy decomposition analysis (EDA) on all possible transition states of the rate-determining step (RDS), the smaller ΔEdef(total) and the larger ΔEint were found in TS3R, resulting in the lowest activation energy (ΔE≠). In other words, TS3R was the most kinetically favorable with the lowest activation barrier. Afterwards, the noncovalent interactions (C–H⋯π and π⋯π) and the steric effects were identified as playing a pivotal role in a favorable transition state for the C–C bond formation. In addition, the frontier molecular orbital (FMO) revealed that the smallest energy gap (5.2836 eV) between the highest occupied orbital (HOMO) and the lowest unoccupied orbital (LUMO) was found in TS3R.


 Please wait while we load your content...
Please wait while we load your content...