Efficient continuous-flow aldehyde tag conversion using immobilized formylglycine generating enzyme†
Abstract
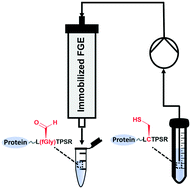
Formylglycine generating enzymes (FGEs) are of growing interest in the biotechnological and pharmaceutical arenas due to their ability to site-specifically install reactive aldehyde functionalities in proteins of interest. In order to overcome the limitations of traditional batch-mode catalysis such as low efficiency and stability, we here report continuous-flow aldehyde tag conversion with high productivity and stability using immobilized FGE. Guided by activity and stability screening, a variant of TcFGE (TcFGEC187A,Y273F, from Thermomonospora curvata) was selected and immobilized via amine coupling onto epoxy-activated Sepharose beads, leading to high immobilization yield (89%, 35.61 mg protein per g of carrier) and activity recovery (47%). Importantly, by developing an in situ strategy for copper cofactor reconstitution, the immobilized FGE can be consecutively reused without further need for cofactor regeneration. Furthermore, the implementation of a continuous-flow biocatalysis system allows for efficient aldehyde tag conversion of the protein substrate with 10 times higher productivity compared to batch reactions. Our work is in response to currently unmet demands in aldehyde tag technology, and establishes a novel platform for site-specific protein modification.


 Please wait while we load your content...
Please wait while we load your content...