Activity adaptability of a DhHP-6 peroxidase-mimic in wide pH and temperature ranges and solvent media†
Abstract
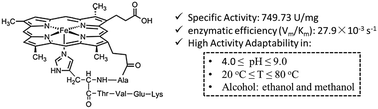
Separating the active units while maintaining the majority of the functions of enzymes seems to be a paradox and therefore challenging, not to mention also improving the adaptability to harsh conditions simultaneously. In this paper, we demonstrate that a well-designed hexapeptide molecule, deuterohemin-β-Ala-His-Thr-Val-Glu-Lys (DhHP-6), adapted from cytochrome c, shows superior peroxidase activity over a wide pH and temperature range. The activity of DhHP-6 was first assessed via three substrates (i.e., 2,2′azinodi(3-ethylbenzthiazoline)-6-sulfonate (ABTS), 1,2,3-trihydroxybenzene (THB) and phenol) with reference to the classically studied examples microperoxidase (MP-11) and horseradish peroxidase (HRP). The environmental adaptability was assessed via three factors: pH, temperature, and the polarity of solvents. In the phenol substrate model reaction, DhHP-6 shows superior specific activity (749.73 U mg−1) and adaptability compared to HRP (311.32 U mg−1). Under the optimum solvent conditions (15% methanol by volume ratio), the enzymatic efficiency (Vm/Km) of DhHP-6 reached 27.9 × 10−3 s−1, 16-times higher than that of HRP (1.81 × 10−3 s−1). The methanol concentration dependent electron paramagnetic spectra show an axial-promoted intermediate process for the phenol catalytic reaction. This work provides a protocol and feasible methodology for the design and fabrication of natural enzyme mimicking small molecules that maintain most of their native activity for better biocatalytic applications in organic pollutant remediation and other related processes.


 Please wait while we load your content...
Please wait while we load your content...