Antimony-doped tin oxide as an efficient electrocatalyst toward the VO2+/VO2+ redox couple of the vanadium redox flow battery†
Abstract
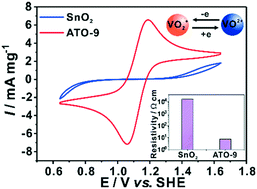
The low electronic conductivity of metallic oxides limits their electrocatalytic activity toward the VO2+/VO2+ redox reaction in a vanadium redox flow battery (VRFB). In this work, antimony-doped tin oxide (ATO) nanoparticles achieve higher electronic conductivity than tin dioxide nanoparticles. In addition, the doped Sb(V) enhances the negative charge on the surface of the SnO2, which contributes to adsorption of vanadium ions on the ATO surface. As a result, the reversibility of the VO2+/VO2+ redox reaction on ATO is much better than that on SnO2. The energy efficiency of the VRFB with optimized ATO nanoparticle loading on graphite felts is improved to 73.09% in comparison with that with pristine graphite felt (61.00%) or SnO2 nanoparticle-coated graphite felt (65.47%) at 300 mA cm−2. Thus, ATO nanoparticle-coated graphite felt is a desirable positive electrode for VRFB applications.


 Please wait while we load your content...
Please wait while we load your content...