Strong Lewis acidic catalysts for C–F bond activation by fluorination of activated γ-Al2O3†
Abstract
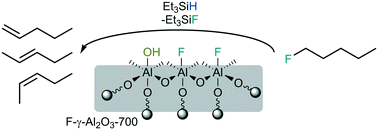
A strong solid Lewis acid catalyst has been successfully obtained by modifying a known procedure of fluorinating γ-aluminium oxide, which was pre-calcined under vacuum. Calcination under vacuum at 700 °C followed by gas phase fluorination with CHClF2 led to various high-surface area materials. The structure and morphology of the catalysts was studied by P-XRD, N2 physisorption and MAS NMR spectroscopy. Their acidic sites were characterized by CD3CN adsorption and DRIFT spectroscopy as well as NH3 TPD measurements. The catalysts exhibit a moderate number of acidic sites, which however possess a very strong Lewis acidic character, and are remarkably active in catalysis, when compared to other fluorinated alumina phases known. They mediate in the presence of a silane with 1-fluoropentane dehydrofluorination reactions, isomerize 1,2-dibromohexafluoropropane and promote dismutation of CHClF2 at room temperature. This outstanding reactivity classifies these novel catalysts as strong solid Lewis acids, comparable with aluminium chlorofluoride or high-surface aluminium fluoride.
- This article is part of the themed collection: 2020 Catalysis Science & Technology Hot Articles


 Please wait while we load your content...
Please wait while we load your content...