Synthetic applications of type II intramolecular cycloadditions
Abstract
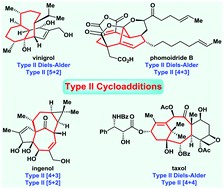
Type II intramolecular cycloadditions ([4+2], [4+3], [4+4] and [5+2]) have emerged recently as an efficient and powerful strategy for the construction of bridged ring systems. In general, type II cycloadditions provide access to a wide range of bridged bicyclo[m.n.1] ring systems with high regio- and diastereoselectivity in an easy and straightforward manner. In each section of this review, an overview of the corresponding type II cycloadditions is presented, which is followed by highlights of method development and synthetic applications in natural product synthesis. The goal of this review is to provide a survey of recent advances in the field covering literature up to 2020. The review will serve as a useful reference for organic chemists engaged in the total synthesis of natural products containing bridged bicyclo[m.n.1] ring systems and provide strong stimulus for invention and further advances in this exciting research field.


 Please wait while we load your content...
Please wait while we load your content...