Visible light-promoted reactions with diazo compounds: a mild and practical strategy towards free carbene intermediates
Abstract
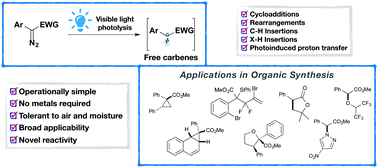
Carbenes are important intermediates in organic chemistry and have been widely applied in various types of organic reactions, ranging from cycloaddition reactions and sigmatropic rearrangements to C–H functionalizations, thus allowing the rapid construction of densely functionalized molecules. Over the past decades, remarkable progress has been achieved in metal-catalyzed carbene transfer reactions. Nevertheless, realizing these transformations under milder and/or greener conditions is still highly desirable. Only recently, visible light-promoted carbene transfer reactions of diazo compounds via free carbene intermediates have emerged as a practical, mild and powerful tool. In this tutorial review, we summarize the latest advances in the area, aiming at providing a clear overview on reaction design, mechanistic scenarios and potential future developments.


 Please wait while we load your content...
Please wait while we load your content...