Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes
Abstract
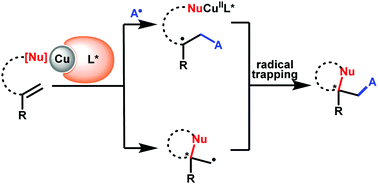
The radical-involved 1,2-difunctionalization of alkenes has developed into a robust tool for preparation of complex organic molecules. Despite significant advances in this area, the catalytic asymmetric version still remains a challenging task mainly due to the difficulty in the stereocontrol of the highly reactive radical intermediates. Recently, owing to the good single-electron transfer ability and coordination with chiral ligands of copper catalysts, remarkable achievements in radical-involved asymmetric alkene difunctionalization have been made via synergistic combination of copper and chiral ligands. This tutorial review highlights the recent progress in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes and the mechanistic scenarios governing the stereocontrol, with an emphasis on utilization of chiral ligands.


 Please wait while we load your content...
Please wait while we load your content...