Equilibria and mesomerism/valence tautomerism of group 4 metallocene complexes
Abstract
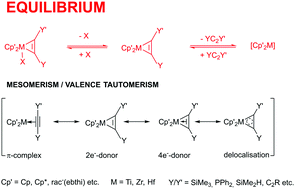
In this account, examples of the influence of equilibrium and mesomerism/valence tautomerism on reactions of selected group 4 metallocene complexes will be discussed. Complexes of bis(trimethylsilyl)acetylene Me3SiC2SiMe3 (mono-functional alkynes: C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) in Cp′2M(η2-Me3SiC2SiMe3) (Cp′ = substituted η5-cyclopentadienyl), of α-di-Ph2P- (hetero-tri-functional: P, C
C) in Cp′2M(η2-Me3SiC2SiMe3) (Cp′ = substituted η5-cyclopentadienyl), of α-di-Ph2P- (hetero-tri-functional: P, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, P) in Cp′2M(η2-Ph2C2PPh2), and of α-mono-SiH- (hetero-bi-functional: C
C, P) in Cp′2M(η2-Ph2C2PPh2), and of α-mono-SiH- (hetero-bi-functional: C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, SiH) and α-di-SiH-substituted alkynes (hetero-tri-functional: SiH, C
C, SiH) and α-di-SiH-substituted alkynes (hetero-tri-functional: SiH, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, SiH) in Cp′2M(η2-Me3SiC2SiMe2H) and Cp′2M(η2-HMe2SiC2SiMe2H), respectively, were considered. Additionally, unusual all-C-metallacycles like metallacyclopentynes Cp′2M[η2-C(R2)–C2–C(R2)–] (formed by coordination of tri-functional trienes: C
C, SiH) in Cp′2M(η2-Me3SiC2SiMe2H) and Cp′2M(η2-HMe2SiC2SiMe2H), respectively, were considered. Additionally, unusual all-C-metallacycles like metallacyclopentynes Cp′2M[η2-C(R2)–C2–C(R2)–] (formed by coordination of tri-functional trienes: C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), five-membered metallacycloallenes Cp′2M[η2-C(R)
C), five-membered metallacycloallenes Cp′2M[η2-C(R)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R)–C(R2)–] (formed by coordination of bi-functional enynes: C
C(R)–C(R2)–] (formed by coordination of bi-functional enynes: C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), metallacyclocumulenes Cp′2M[η2-C(R)
C), metallacyclocumulenes Cp′2M[η2-C(R)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R)–] (formed by coordination of bi-functional diynes: C
C(R)–] (formed by coordination of bi-functional diynes: C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, C
C, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), and seven-membered metallacyclocumulenes Cp′2M[η2-C(R)
C), and seven-membered metallacyclocumulenes Cp′2M[η2-C(R)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C2R)–C(R)
C(C2R)–C(R)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R)–] (formed by coordination of two bi-functional diynes: C
C(R)–] (formed by coordination of two bi-functional diynes: C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, C
C, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), with M = Ti, Zr, and Hf were selected as examples for considerations of equilibrium and mesomerism/valence tautomerism. On the basis of spectroscopic investigations, their molecular structures and selected calculations, it is discussed how the different interpretations of the equilibrium and mesomerism/valence tautomerism are in line with observed reactivity patterns.
C), with M = Ti, Zr, and Hf were selected as examples for considerations of equilibrium and mesomerism/valence tautomerism. On the basis of spectroscopic investigations, their molecular structures and selected calculations, it is discussed how the different interpretations of the equilibrium and mesomerism/valence tautomerism are in line with observed reactivity patterns.


 Please wait while we load your content...
Please wait while we load your content...