Transition-metal-catalyzed reactions involving reductive elimination between dative ligands and covalent ligands†
Abstract
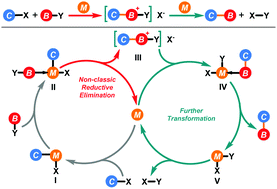
Reductive elimination is a crucial bond-forming elementary reaction in various transition-metal mediated reactions. Apart from the well-developed classic reductive elimination, the non-classic reductive elimination occurring between a covalent ligand and a dative ligand, which has been known for over 50 years, has gradually attracted much attention from the organic community. By avoiding pπ–dπ repulsion between the filled metal d-orbital and the filled ligand p-orbital and forming a cationic-type molecule, non-classic reductive elimination could facilitate many catalytic reactions that were difficult to be realized via classic reductive elimination. In this review, transition-metal catalyzed C–P, C–S, C–N, and C–C bond-forming reactions with non-classic reductive elimination as the key elementary step are summarized.


 Please wait while we load your content...
Please wait while we load your content...