Visible light-promoted ring-opening functionalization of three-membered carbo- and heterocycles
Abstract
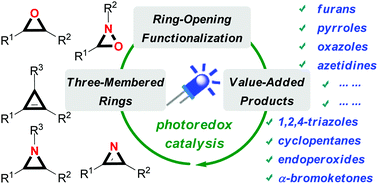
Due to their inherent ring strain, three-membered carbocyclic- and heterocyclic ring structures are versatile synthetic building blocks. Traditional ring-opening methods of these molecules require the use of thermolysis, acid catalysts or transition-metals via ionic reaction pathways. Recently, visible light-induced photoredox catalysis has emerged as a powerful platform for initiating new chemical transformations. In this tutorial review, the synthetic and mechanistic aspects of visible light-promoted ring-opening functionalization of three-membered carbo- and heterocycles are highlighted. By using these strategies, a variety of ring-opening functionalization products, including biologically important carbo- and heterocycles, can be efficiently accessed in a high chemo- and regioselective manner.


 Please wait while we load your content...
Please wait while we load your content...