Palladium-catalyzed oxidative dehydrogenative carbonylation reactions using carbon monoxide and mechanistic overviews
Abstract
Carbon monoxide, which is an abundant and inexpensive carbonyl source, has been widely applied to synthesize carbonyl-containing compounds, for example ketones, esters, and amides. These types of compounds are ubiquitous in natural products, pharmaceuticals, as well as in functional materials. This review focuses on the palladium-catalyzed dehydrogenative C–H/X–H (X = C, N, O) carbonylation transformations under oxidative conditions. The related C–H bonds here include C(sp)–H, C(sp2)–H, and C(sp3)–H bonds. From a step- and atom-economy perspective, transition metal-catalyzed oxidative dehydrogenative C–H/X–H carbonylation reactions with CO constitute one of the most efficient strategies for the construction of versatile carbonyl groups, without the requirement of pre-functionalized substrates.
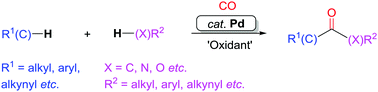


 Please wait while we load your content...
Please wait while we load your content...