Infrared action spectroscopy of nitrous oxide on cationic gold and cobalt clusters†
Abstract
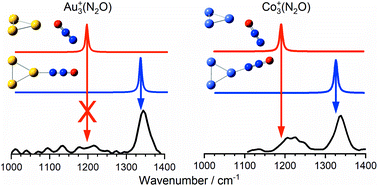
Understanding the catalytic decomposition of nitrous oxide on finely divided transition metals is an important environmental issue. In this study, we present the results of a combined infrared action spectroscopy and quantum chemical investigation of molecular N2O binding to isolated Aun+ (n ≤ 7) and Con+ (n ≤ 5) clusters. Infrared multiple-photon dissociation spectra have been recorded in the regions of both the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1000–1400 cm−1) and N
O (1000–1400 cm−1) and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (2100–2450 cm−1) stretching modes of nitrous oxide. In the case of Aun+ clusters only the ground electronic state plays a role, while the involvement of energetically low-lying excited states in binding to the Con+ clusters cannot be ruled out. There is a clear preference for N-binding to clusters of both metals but some O-bound isomers are observed in the case of smaller Con(N2O)+ clusters.
N (2100–2450 cm−1) stretching modes of nitrous oxide. In the case of Aun+ clusters only the ground electronic state plays a role, while the involvement of energetically low-lying excited states in binding to the Con+ clusters cannot be ruled out. There is a clear preference for N-binding to clusters of both metals but some O-bound isomers are observed in the case of smaller Con(N2O)+ clusters.


 Please wait while we load your content...
Please wait while we load your content...