Using anion photoelectron spectroscopy of cluster models to gain insights into mechanisms of catalyst-mediated H2 production from water
Abstract
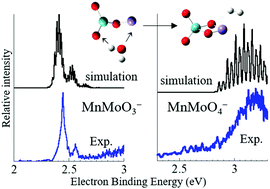
Metal oxide cluster models of catalyst materials offer a powerful platform for probing the molecular-scale features and interactions that govern catalysis. This perspective gives an overview of studies implementing the combination of anion photoelectron (PE) spectroscopy and density functional theory calculations toward exploring cluster models of metal oxides and metal-oxide supported Pt that catalytically drive the hydrogen evolution reaction (HER) or the water–gas shift reaction. The utility in the combination of these experimental and computational techniques lies in our ability to unambiguously determine electronic and molecular structures, which can then connect to results of reactivity studies. In particular, we focus on the activity of oxygen vacancies modeled by suboxide clusters, the critical mechanistic step of forming proximal metal hydride and hydroxide groups as a prerequisite for H2 production, and the structural features that lead to trapped dihydroxide groups. The pronounced asymmetric oxidation found in heterometallic group 6 oxides and near-neighbor group 5/group 6 results in higher activity toward water, while group 7/group 6 oxides form very specific stoichiometries that suggest facile regeneration. Studies on the trans-periodic combination of cerium oxide and platinum as a model for ceria supported Pt atoms and nanoparticles reveal striking negative charge accumulation by Pt, which, combined with the ionic conductivity of ceria, suggests a mechanism for the exceptionally high activity of this system towards the water–gas shift reaction.
- This article is part of the themed collections: PCCP Perspectives and 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...