Vibrational circular dichroism towards asymmetric catalysis: chiral induction in substrates coordinated with copper(ii) ions†
Abstract
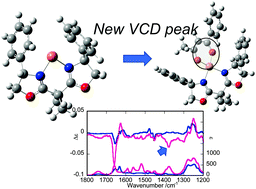
Cu(II) complexes containing RR- or SS-2,2′-isopropylidene-bis(4-phenyl-2-oxazoline) (denoted as [Cu(RR- or SS-oxa)]2+) are known to catalyse many asymmetric organic reactions. Herein, the source of enantioselectivity was investigated by vibrational circular dichroism (VCD) spectroscopy. An achiral β-diketonato ligand (denoted as LH), such as 1-phenyl-1,3-butanedione and dibenzoylmethane, was added to form [Cu(RR- or SS-oxa)L]+. Clear VCD peaks were obtained from a CDCl3 solution of [Cu(RR- or SS-oxa)]2+ or [Cu(RR- or SS-oxa)L]+ at 1000–1800 cm−1. It is to be noted that when LH was coordinated, a new VCD peak appeared at ∼1380 cm−1, which was assigned to the C–O asymmetric stretching vibration of L−. Theoretical simulation helped rationalise the results in terms of the transformation of coordinated L− into a twisted chiral form. The extent of steric control within the coordination sphere was demonstrated, revealing the first step for enantioselectivity during catalysis.


 Please wait while we load your content...
Please wait while we load your content...
